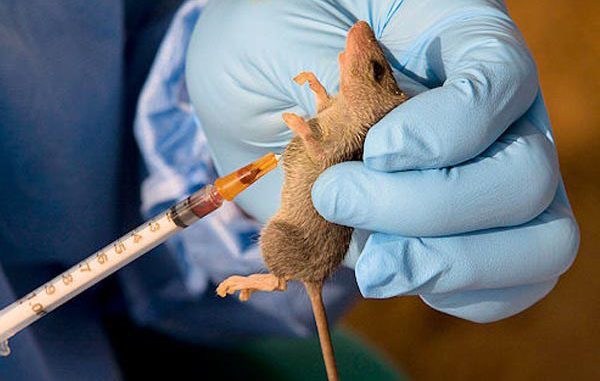
IT is heartening that efforts are ongoing to develop a vaccine for Lassa fever. An American biotechnology company, INOVIO, which is spearheading the research, noted that the first participant in Lassa vaccine trial had been dosed in a Phase 1B clinical trial for INO-4500, its DNA vaccine candidate for Lassa fever. The clinical trial in progress in Ghana is reportedly focusing on offering precisely-designed DNA medicines to treat and protect people from infectious diseases and cancer.
Lassa fever, according to the United States Centres for Disease Control and Prevention, is an animal-borne, or zoonotic, acute viral illness. It is endemic in parts of West Africa, including Sierra Leone, Liberia, Guinea and Nigeria. The illness was discovered in 1969 and is named after the town in Nigeria where the first cases occurred. The CDC says an estimated 100,000 to 300,000 infections of Lassa fever occur annually, with approximately 5,000 deaths. The World Health Organisation describes it as a viral haemorrhagic fever transmitted to humans through contact with food or household items contaminated with rodent urine or faeces.
With the vaccine, the annual scourge appears to be on its way out. The Phase 1B clinical trial (LSV-002), ongoing at the Noguchi Memorial Institute for Medical Research in Accra, Ghana, is the first vaccine clinical trial for Lassa fever. INO-4500 was also the first vaccine candidate for Lassa fever to enter human trials stage.
The disease is prevalent in Nigeria, having been first discovered in the late 1960s and cases annually increase during the dry season between December and April. Last year, the country recorded 472 laboratory-confirmed cases, including 70 deaths in 26 of the country’s 36 states and the Federal Capital Territory. The WHO noted that 75 per cent of the cases were reported from three states: Edo (167 cases), Ondo (156 case) and Ebonyi (30 cases). Other states, which also battled the disease during that year included Bauchi, Taraba, Kogi, Kano, Delta, Borno, Plateau, Rivers, Enugu, Kaduna, Benue, Oyo, Anambra, Adamawa, Sokoto, Osun, Abia, Katsina, Kebbi, Ogun, Gombe and Nasarawa, and the FCT.
The Nigerian Institute of Medical Research was established to, among other functions, improve public health and national development, including collaborating with several institutions nationally and internationally to facilitate an enabling environment for medical research. The tweet by the Director-General, Nigeria Centre for Disease Control, Chikwe Ihekweazu, while lauding the novel efforts for Lassa fever vaccine, emphasised the need for increased collaborative efforts to improve the health indices. He added, “We have worked very hard with the WHO, CEPI vaccines, ACEGID, BNITM_de and many others to put this on the global health agenda. We will keep pushing.” Medical research is funded by various entities, including government, patient and disease groups, and industry.
INOVIO is said to be advancing INO-4500 with funding from the Coalition for Epidemic Preparedness Innovations, a global partnership leveraging funding from public, private, philanthropic and civil society organisations to support research projects to develop vaccines against emerging infectious diseases. INOVIO previously received a $56m grant from CEPI in 2018, with which the company is developing vaccine candidates for Lassa fever and the Middle East Respiratory Syndrome. As INOVIO and CEPI join forces to make a vaccine available as soon as possible for emergency use as a stockpile product post-Phase 2 testing, it is crucial for Nigeria to look inwards by using local research to curb tropical and infectious diseases.
Regrettably, medical research funding is low in Nigeria. Figures from the Expanded Special Project for Elimination of Neglected Tropical Diseases (a WHO AFRO region project) for 2019 indicated that the country needed to do more in that area of funding. In 2019, Nigeria’s treatment coverage for five of the 20 neglected tropical diseases covered by the WHO showed that elephantiasis was 62 per cent, blinding trachoma 67 percent, internal worms 76 percent, schistosomiasis (commonly called bilharzia) 99 per cent and river blindness 80 per cent. Of these figures, 96.96 million received treatment while 37.59 million did not receive treatment in Nigeria in 2019.
In the US, according to reports, the Federal Government provides core sources of support for basic biomedical research and development. In general terms, 64 per cent of all applied biomedical R&D funding comes from within the industry, while the Federal Government provides about 22 per cent of the funding. For elephantiasis, treatment coverage decreased from 65 per cent in 2018 to 62 per cent in 2019, while 134.55 million needed treatment, 82.99 million received treatment. In the same year, for blinding trachoma, treatment coverage increased from 53 per cent in 2018 to 67 per cent in 2019. About 13.91 million needed treatment and 9.3 million received treatment. Internal worms recorded an increase in treatment coverage from 44 per cent in 2018 to 76 per cent in 2019. While the pre- and school-aged children needing treatment were 45.21 million, about 34.35 million received treatment. For bilharzia, treatment coverage increased from 67 per cent in 2018 to 99 per cent in 2019.
Also, school-aged children needing treatment for the disease were 17.13 million and those who received treatment totalled 16.93 million. Treatment coverage of increase from 79 per cent in 2018 to 80 per cent in 2019 was recorded for river blindness. For the disease, those needing treatment were 50.57 million and those who received treatment were 4.7 million. Apparently, the time is now for the country’s health research institutes to galvanise research findings and reliability to stem the tide of tropical diseases in the country.
END

Be the first to comment