The Netherlands has become the latest country to suspend use of the Oxford-AstraZeneca coronavirus vaccine over concerns about possible side effects.
The Dutch government said the move, which will last until at least 29 March, was a precaution.
The Republic of Ireland earlier made a similar decision over reports of blood clotting events in adults in Norway.
But the World Health Organization says there is no link between the jab and an increased risk of developing a clot.
The European Medicines Agency (EMA) – which is currently carrying out a review into incidents of blood clots – says the vaccine’s benefits continue to outweigh its risks.
Denmark, Norway, Bulgaria, Iceland and Thailand have already halted their use of the AstraZeneca vaccine.
What measures did the Dutch government take?
In a statement, the Dutch government said it was acting out of precaution following reports from Denmark and Norway of possible serious side effects.
“We can’t allow any doubts about the vaccine,” Dutch Health Minister Hugo de Jonge said.
“We have to make sure everything is right, so it is wise to pause for now.”
Why is the EU having vaccine problems?
EU closes ranks over Covid surge and vaccine delays
Sunday’s decision will now cause delays in the Dutch vaccination programme.
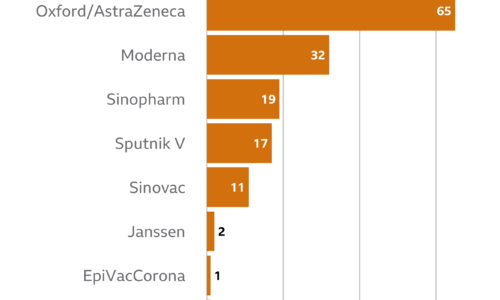
The authorities had pre-ordered 12 million doses of AstraZeneca, with nearly 300,000 jabs scheduled in the next two weeks.
What did AstraZeneca say?
In a statement, AstraZeneca said there was no evidence of an increased risk of clotting due to the vaccine.
It said that across the EU and United Kingdom there had been 15 events of deep-vein thrombosis (DVT) and 22 events of pulmonary embolism reported among those vaccinated.
“Around 17 million people in the EU and UK have now received our vaccine, and the number of cases of blood clots reported in this group is lower than the hundreds of cases that would be expected among the general population,” said Ann Taylor, the firm’s chief medical officer.
“The nature of the pandemic has led to increased attention in individual cases and we are going beyond the standard practices for safety monitoring of licensed medicines in reporting vaccine events, to ensure public safety.”
While vast numbers of people are being vaccinated at pace around the world, some of them will still get sick with other things unrelated to the vaccine.
These pauses for the AstraZeneca vaccine are not because it is unsafe to give. It’s to allow time for experts to explore why a small number of people who were recently give the shot also developed blood clots.
When an illness occurs shortly after vaccination, it is right to question whether the shot might have contributed in any way.
There is no indication or evidence, however, that the vaccine was linked or responsible.
In the UK, more than 11 million people have already received at least one dose of the vaccine and there has been no sign of excess deaths or blood clots occurring. Europe’s drug regulator has also backed the vaccine, saying its benefits are clear. Covid can be deadly and vaccination saves lives.



Be the first to comment