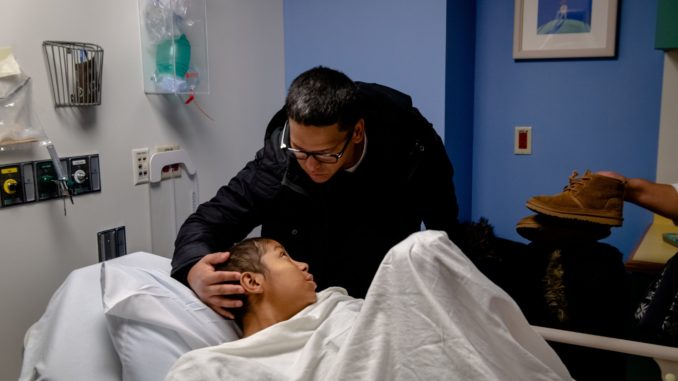
BOSTON — Helen Obando, a shy slip of a girl, lay curled in a hospital bed in June waiting for a bag of stem cells from her bone marrow, modified by gene therapy, to start dripping into her chest.
The hope was that the treatment would cure her of sickle cell disease, an inherited blood disorder that can cause excruciating pain, organ damage and early death.
Sedated with Benadryl to prevent an allergic reaction to the garlicky-smelling preservative in the drip, Helen, who at 16 was the youngest person ever to undergo the therapy, was sound asleep for the big moment.
“Wake up,” her younger brother, Ryan, said, shaking her leg so she could push the button to start the drip. But she could not be roused, so he pushed it himself.
Helen’s family and the medical team watched in awe as the pale pink solution of cells flowed in through her left subclavian vein.
Meet Helen, the youngest patient to receive a breakthrough gene therapy in an attempt to cure her sickle cell disease.
It was a critical moment in medical science.
For more than a half century, scientists have known the cause of sickle cell disease: A single mutation in a gene turns red blood cells into rigid crescent or sickle shapes instead of soft discs. These misshapen cells get stuck in veins and arteries, blocking the flow of blood that carries life-giving oxygen to the body and causing the disease’s horrifying hallmark: episodes of agony that begin in babyhood.
Millions of people globally, a vast majority of them Africans, suffer from sickle cell disease. Researchers have worked for decades on improving treatment and finding a cure, but experts say the effort has been hindered by chronic underfunding, in part because most of the estimated 100,000 people in the United States who have the disease are African-American, often poor or of modest means.
The disease also affects people with southern European, Middle Eastern or Asian backgrounds, or those who are Hispanic, like Helen.
This is the story of two quests for a sickle cell cure — one by the Obando family, and one by a determined scientist at Boston Children’s Hospital, Dr. Stuart Orkin, 73, who has labored against the disease since he was a medical resident in the 1970s.
Like many others affected by sickle cell, the Obando family faced a double whammy: not one but two children with the disease, Helen and her older sister, Haylee. They lived with one hope for a cure, a dangerous and sometimes fatal bone marrow transplant usually reserved for those with a healthy sibling as a match. But then they heard about a potential breakthrough: a complex procedure to flip a genetic switch so the body produces healthy blood.
Scientists have been experimenting with gene therapy for two decades, with mixed success. And it will be years before they know if this new procedure is effective in the long term. But if it is, sickle cell disease could be the first common genetic disorder to be cured by manipulating human DNA.
“It’s an exhilarating success story for those of us who have waited and hoped for this day,” said Dr. Francis S. Collins, the director of the National Institutes of Health.
Curing someone as young as Helen would be especially significant. Sickle cell is progressive, and every year, it wreaks more devastating damage to her body.
If the therapy shuts down the disease in her and another teenager in the same clinical trial at Boston Children’s, scientists will soon begin testing it in even younger children.
Two other gene therapy trials for sickle cell, using different methods, are also underway in the United States. One also aims to flip the genetic switch, while the other adds a new gene. If approved, such cures would almost certainly cost $1 million or more, experts say, raising questions about affordability.
For now, Helen’s therapy is covered by federal research grants. But the hospital has licensed patents it develops to the biotech firm Bluebird Bio, giving the company the option to sell the treatment and pay royalties to the hospital. Bluebird Bio declined to comment on the agreement or speculate on the price of the therapy.
Four weeks after the infusion of stem cells, Helen was strong enough to be discharged. Her bald head wrapped in a pink scarf and held high, she walked out with a mask over her nose and mouth to protect her from germs. She turned the corner in the hallway and was greeted by 30 doctors and nurses blowing bubbles as “Girl on Fire” by Alicia Keys played.
At home, in Lawrence, Mass., on a sofa with her mother by her side, she put a hand over her eyes and started to sob. Ryan enveloped her in a hug.
She and her family wondered: Would it work? Was her suffering really over?
A family’s nightmare
Sheila Cintron, 35, and Byron Obando, 40, met when she was in the eighth grade and he was a high school senior. They fell in love. Haylee, their first child, was born in 2001, when Sheila was 17.
When a newborn screening test showed that Haylee had the disease, her father asked, “What’s sickle cell?”
They soon found out.
As the family gathered for her first birthday party, Haylee started screaming inconsolably. They rushed her to the hospital. It was the first of many pain crises.
Doctors warned the parents that if they had another baby, the odds were one in four that the child would have sickle cell, too. But they decided to take the chance.
“I was young, naïve,” Ms. Cintron said.
Less than two years later, Helen was born. As bad as Haylee’s disease was, Helen’s was much worse. When she was 9 months old, a severe blockage of blood flow in her pelvis destroyed bone. She screamed with pain, no longer able to sit up.
At age 2, her spleen, which helps fight bacterial infections, became dangerously enlarged because of blocked blood flow. That can cause severe anemia, so doctors surgically removed the organ.
END

Be the first to comment